
Behind-the-scenes: Production of Viral Vector Vaccines
The industry of vaccines used to be small. However, in recent years, the demand for vaccines has grown exponentially. This is due to a number of factors, including the outbreak of several new diseases and the increasing global population. As a result, the industry of vaccines has grown significantly.
This industry is now worth billions of dollars and employs thousands of people around the world. However, this growth has also created a demand for experts in the field of vaccines. These experts are responsible for research and development, as well as manufacturing and marketing.
The demand for experts in this field is only expected to grow in the coming years. This is due to the continued growth of the industry and the increasing global population. As a result, those with experience in this field will be in high demand.
Companies not only use efficient and effective technology but also employ highly skilled professionals who work to improve and leverage existing processes to ensure product quality. The process of creating a vaccine requires many steps and manipulations in a controlled environment. This includes temperature and humidity.
Let’s continue the series by focusing on viral vector production, one of the most common vaccines manufacturing applications, their contribution towards eradicating COVID-19 and the experts behind it.
Production of Viral Vectors to Vaccines
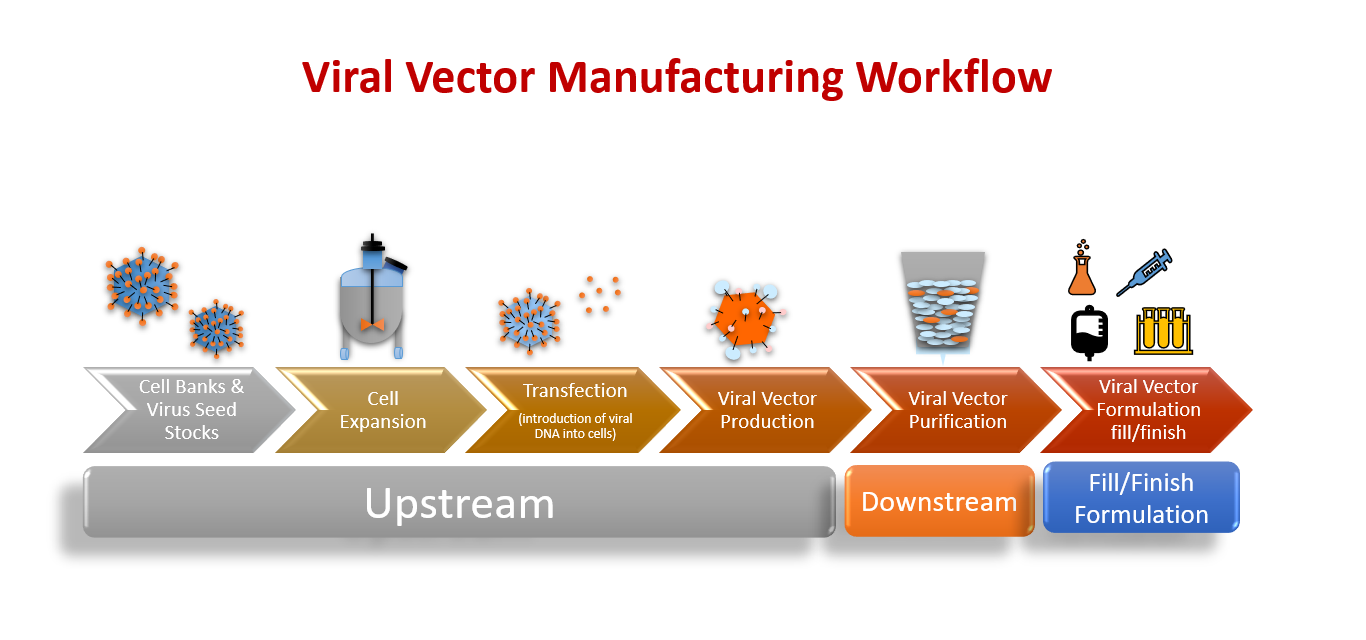
Biosecurity is essential for the production of vaccine-grade viruses. Low pathogenic viruses are therefore often chosen. It is crucial to understand the epidemiological and virological characteristics of viral vectors before using them. Each vector has its own unique property, which is determined by the virus. Because they can incite strong immune responses against foreign antigens, Adenovirus is the most commonly used vector.
This process requires the use of state-of-the-art technology and strict control according to Good Manufacturing Practices guidelines (GMP). This process is campaign-based. Only one product is made at a given time. Disposable materials and closed systems are used frequently to maintain high quality. Open processes are carried out in biosafety cabinets equipped with HEPA filters. The manufacturing facility exhausts directly.
Viral Vector Production Suite Area

The production area has HVAC technology and a single-pass ventilation system. This facility has a restricted passage that can be controlled electronically and pressured airlocks to restrict personnel and material entry. HEPA filters are installed in the area. Gas supplies (CO2, O2, and N2) are also available. Due to process security and sensitivity, the facility needs 24/7 monitoring.
Different viruses have different production processes. This means that each virus requires its own equipment and facilities. It takes more than 20 scientists to make sure master cells are ready for the production process. Everything begins at the laboratory on a small scale. After large quantities of bacteria or viruses have been grown, the next step is to isolate, purify, attenuate or inactivate them, depending on what vaccine they are. Each step requires specific equipment and reagents. These steps can also increase costs.
One example is that poliovirus can be grown in dishes, which require different handling. However, bacteria-based vaccines can be grown in large bioreactors. To prevent live pathogens from escaping, it is important to take strict precautions.
Process
Scientists map the genetic material that will be used to deliver the treatment to the cells/host once the bad virus has been removed from the capsule. Once the master virus vector has been removed from the capsule, it is ready for large-scale vaccine manufacturing for formulation and vial fill process. Once the formulation has been determined, it is necessary to determine the vaccine volume (cc), to be given to patients. This genomic isolation can also be used for gene therapy plasmid applications.
We learned this from “What Are Viral Vectors and How Do They Function?” Contract Manufacturing Operations (CMOs), and Contract Development Manufacturing Operations (CDMOs), are large-scale facilities that produce vaccines. It is important to understand the importance of both upstream and downstream steps in production.
ALSO READ: What is Medical Treatment Visa and Everything You Need to Know AboutMedical Treatment Visa




